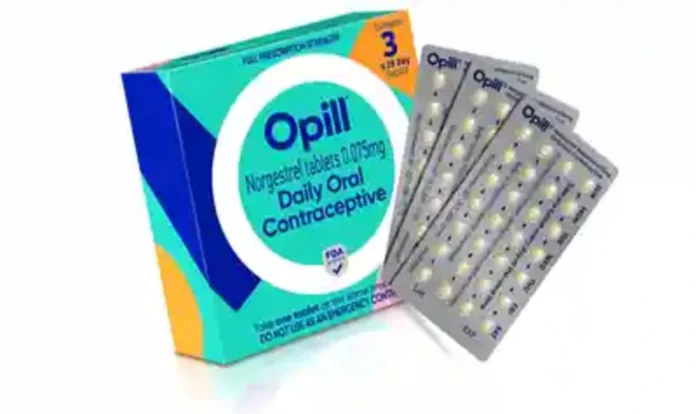
In a groundbreaking move, the United States Food and Drug Administration (FDA) has granted approval for Opill, a revolutionary birth control pill, to be made available without a prescription. This historic decision marks a significant milestone that has the potential to reshape the landscape of contraception access nationwide. Opill, manufactured by Perrigo Company, is set to redefine the standard for over-the-counter birth control methods, surpassing traditional options like condoms, spermicides, and various nonprescription alternatives.
Dublin-based Perrigo Company has announced its plans to make Opill available in stores and online retailers throughout the United States starting in early 2024. While the cost of the medication has not been disclosed, Perrigo’s global vice president for women’s health, Frédérique Welgryn, has emphasized the company’s commitment to ensuring its accessibility and affordability for women and people of all ages. Welgryn further revealed that Perrigo would implement a consumer assistance program to provide the pill at no cost to certain women, making it an even more accessible option. Leading medical authority, Dr. Patrizia Cavazzoni, has expressed her profound excitement about the FDA’s recent approval.
This decision heralds a new era for millions of individuals across the United States, granting them unprecedented access to a nonprescription daily oral contraceptive. Dr. Cavazzoni underscored the importance of using daily oral contraception correctly, highlighting its remarkable safety and effectiveness. She emphasized that Opill outperforms currently available nonprescription contraceptive methods, offering superior efficacy in preventing unintended pregnancies. The initiative to expand access to a nonprescription birth control pill has garnered significant support from influential figures in the field of reproductive health, esteemed adolescent health experts, and reputable organizations such as the American Medical Association, the American College of Obstetricians and Gynecologists, and the American Academy of Family Physicians.
Opill, also known as a “mini-pill,” differentiates itself from other contraceptive methods by containing only one hormone, progestin, as opposed to combination pills that contain both progestin and estrogen. Cadence Health, another company manufacturing combination pills, is reportedly in discussions with the FDA to seek over-the-counter status as well. While concerns were raised about potential risks for individuals with medical conditions that should preclude them from taking birth control pills, FDA analysts deemed the risk to be very low as long as users read and adhere to the labeling instructions. Advisory committee members highlighted that patients with breast cancer, for whom hormonal contraception is contraindicated, are typically advised by their doctors to avoid birth control pills.
Perrigo’s report on the study participants demonstrated high adherence rates, with Opill being taken on 92.5 percent of the recommended days. The incidence of pregnancy while using Opill was minimal, with only six cases reported among the 955 participants. The introduction of over-the-counter birth control pills has the potential to transform the field of contraception, providing enhanced accessibility, particularly for communities that have historically faced barriers. This milestone represents a significant step forward in empowering individuals to take control of their reproductive health and make informed choices.